How wearables are helping in the fight against Covid-19
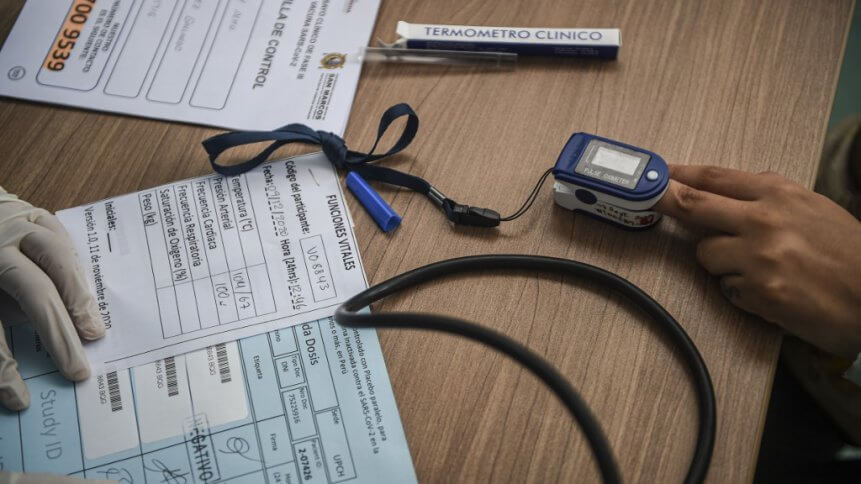
- Remote monitoring, telehealth applications, and wearable tech were instrumental to enabling the decentralized clinical trials that accelerated vaccine timetables
COVID-19 vaccinations have begun in earnest this year, with the UK, US, and other nations rolling out vaccines from big pharma firms like Pfizer, AstraZeneca, and Moderna. Usually, vaccines for new contagions undergo strenuous human clinical trials to ascertain their safety and viability for human consumption.
But the urgency presented by the highly-infectious COVID-19 strains have necessitated an accelerated timetable for vaccines as the number of active cases worldwide approaches the 100 million mark. Nevertheless, clinical trials for other drugs could take years before an effective vaccine is distributed widely, prompting questions on the efficacy of the speedy vaccine introductions to contain the coronavirus spread.
A new report from Oracle Health Sciences and Informa Pharma Intelligence attempts to determine exactly how the pandemic was affecting clinical trial processes, as well as to identify the key challenges and opportunities involved in not only the breakthrough research, but in the technologies that is helping move up the vaccination schedule.
The report surveyed 252 professionals worldwide who are involved in clinical trials at biopharmaceutical and medical device companies. Chief among their input was the role of innovation to accelerate clinical trials, with researchers active in different countries separated by social distancing measures in addition to other priorities.
This meant that there were a lot of decentralized trials. Instead of researchers studying together and cross-referencing their findings in a centralized control laboratory, the pandemic – along with new guidance from the US’ Federal Drug Administration (FDA) – has resulted in large numbers of companies opting to adopt remote approaches. The survey found that 76% of respondents noted at least some of their trials are decentralized, while 38% of respondents noted more than half of their trials were decentralized.
There are many steps involved with a move to decentralized clinical trials. The most common step being taken by companies is the adoption of patient-facing technologies or alternatives, which was cited by 64% of respondents.
Over a quarter of survey respondents highlighted issues they faced with remote trials, including with how the data is collected, issues with retaining the same patients over the trial period, increased deviations of data that were not collected as per specified protocols, as well as delayed peer and medical board reviews.
As COVID was impacting trials, it was also impacting strategies for running clinical trials. The biggest strategy change involved virtual trials. The survey found 46% of respondents were implementing or planning to implement decentralized trials.
Remote data collection goes hand-in-hand with decentralized trials. The majority of respondents (67%) noted they have already implemented remote data collection or are planning to do so. The means of implementing remote data collection varies, with 57% noting they are using patient apps, 49% citing ePRO, 45% are planning to use wearables/devices, while others still mention monitors and mobile health (mHealth) applications.
YOU MIGHT LIKE

Do patients want more digital health services?
24% state they are already using wearable and remote monitoring technologies, with another 13% planning to do so within the next 6 months. Many respondents noted the benefits of real-time monitoring tech, with 64% noting the added convenience for the patient, who did not have to encumbered by additional medical monitoring equipment to assess the effects of the vaccine trial.
The immediacy of the real-time data and insights delivered by wearables was appreciated by at least half (52%) of those surveyed, along with time and resource savings for the testing site staff (45% of those canvassed), convenience for site staff (30%), and also time/resource savings for companies sponsoring the clinical testing (28%).
But there are also downsides of wearables and remote monitoring, primarily to do with the way data is collected and interpreted, but also concerning cost and regulatory considerations. More than 40% of respondents were concerned about the quality of data gathered, followed by data protection/privacy concerns, a lack of standardization in data methodology, and a lack of harmonization or unifying traits of the data collected.
What it means for businesses
The Oracle and Informa study underscored how remote monitoring, telehealth to keep in contact with patients, and wearable tech were instrumental to enabling virtual clinical trials. Without these innovations conducting trials across borders would have been difficult, if not impossible with the pandemic still raging.
While concerns remain over cost and regulatory guidance around decentralized clinical trials, businesses can surely identify with how virtualized trials correspond with the challenges and opportunities of remote working culture that was likewise accelerated as a result of the pandemic.
In particular, the adoption of digitalized innovations to maintain communications is very similar, as is the use of remote monitoring and smart wearable tech, which is useful in the workplace as well, particularly in a pandemic where they can conveniently and cost-efficiently help monitor employee vitals – even while they are working remotely.








